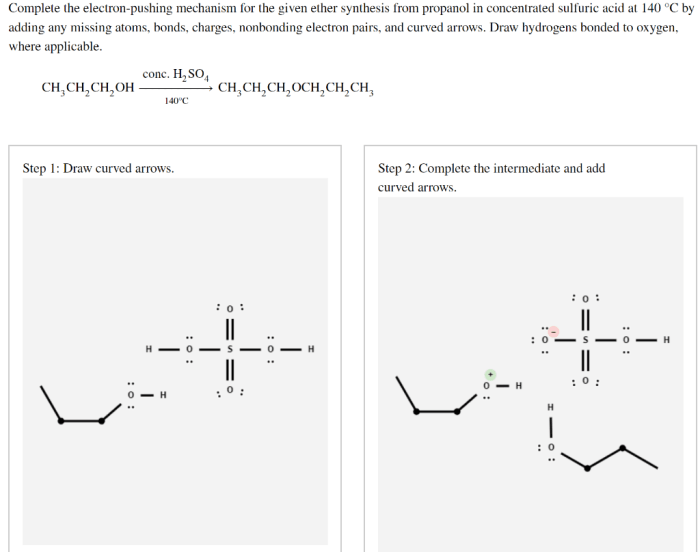
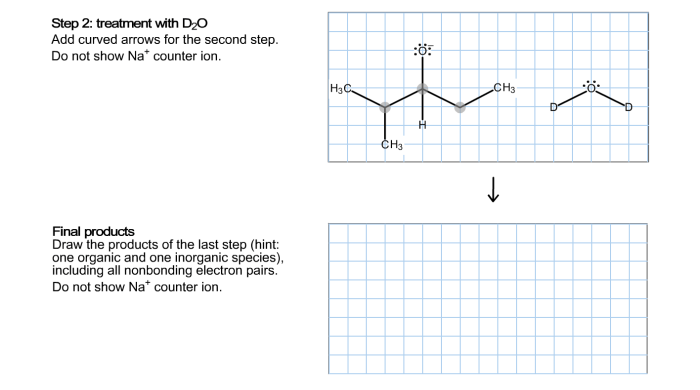
Complete the electron pushing mechanism for the given ether – Delving into the realm of ether chemistry, we embark on a journey to unravel the intricacies of the electron-pushing mechanism for ether formation. This fundamental concept serves as the cornerstone for understanding the reactivity and applications of these versatile compounds.
Electron-pushing mechanisms provide a visual representation of the movement of electrons during a chemical reaction, allowing us to track the flow of charge and predict the outcome of the process. In the case of ether formation, the electron-pushing mechanism illuminates the key steps involved in the transformation of an alcohol into an ether.
Electron-Pushing Mechanism for Ether Formation: Complete The Electron Pushing Mechanism For The Given Ether
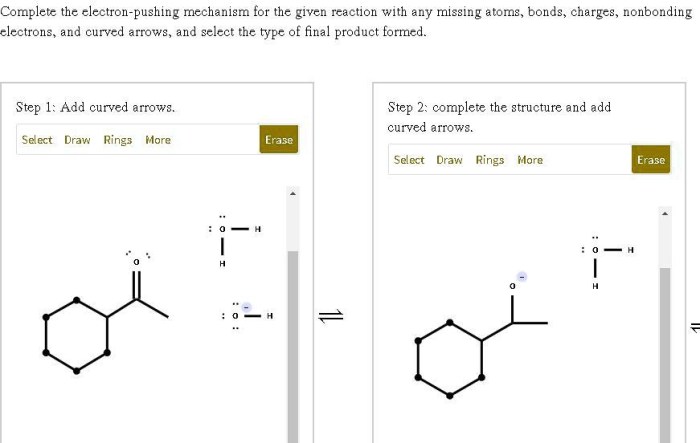
Ethers are organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. They are common solvents and are also used in the synthesis of other organic compounds. Ethers can be formed by a variety of methods, but one of the most common is the nucleophilic substitution reaction of an alcohol with an alkyl halide.
The electron-pushing mechanism for ether formation is a step-by-step process that shows how the electrons move during the reaction. The first step is the nucleophilic attack of the alcohol on the alkyl halide. This forms a new bond between the oxygen atom of the alcohol and the carbon atom of the alkyl halide.
The second step is the departure of the halide ion. This leaves the oxygen atom of the alcohol with a positive charge. The third and final step is the formation of the ether bond. This occurs when the oxygen atom of the alcohol attacks the carbon atom of the alkyl halide, forming a new bond between the two atoms.
Variations in Electron-Pushing Mechanisms
There are several variations of the electron-pushing mechanism for ether formation. One variation is the Williamson ether synthesis. This reaction uses an alkoxide ion as the nucleophile instead of an alcohol. Another variation is the alkylation of alkoxides. This reaction uses an alkyl halide as the electrophile instead of an alcohol.
The electron-pushing mechanisms for these variations are similar to the mechanism for the nucleophilic substitution reaction of an alcohol with an alkyl halide. However, there are some key differences. For example, in the Williamson ether synthesis, the alkoxide ion is a stronger nucleophile than an alcohol.
This means that the reaction proceeds more quickly.
Applications of Ether Formation, Complete the electron pushing mechanism for the given ether
Ether formation is a versatile reaction that can be used to synthesize a variety of organic compounds. Ethers are used as solvents, fragrances, and pharmaceuticals. They are also used in the protection of hydroxyl groups in organic synthesis.
One example of the use of ethers in organic synthesis is the synthesis of diethyl ether. Diethyl ether is a common solvent that is used in a variety of reactions. It is also used as an anesthetic.
Another example of the use of ethers in organic synthesis is the protection of hydroxyl groups. Hydroxyl groups are often protected with ethers to prevent them from reacting with other reagents. This can be important in the synthesis of complex organic molecules.
FAQ Summary
What is the role of the nucleophile in ether formation?
The nucleophile attacks the electrophilic carbon of the alkyl halide, initiating the electron-pushing mechanism and leading to the formation of the ether.
How does the Williamson ether synthesis differ from the alkylation of alkoxides?
In the Williamson ether synthesis, an alkoxide ion acts as the nucleophile, while in the alkylation of alkoxides, an alcohol is deprotonated to generate the nucleophilic alkoxide ion.