Embark on a journey through Chapter 11 Stoichiometry Study Guide, an indispensable resource for comprehending the fundamental principles of chemical proportions. This comprehensive guide will illuminate the intricacies of stoichiometry, empowering you to navigate the complexities of chemical reactions with confidence and precision.
Delve into the heart of stoichiometry, unraveling the concepts that govern the quantitative relationships between reactants and products. Explore the mole concept, a cornerstone of stoichiometric calculations, and uncover the secrets of balancing chemical equations, ensuring that every atom finds its rightful place.
Stoichiometry Basics: Chapter 11 Stoichiometry Study Guide

Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It involves using balanced chemical equations to determine the exact amounts of reactants and products that are involved in a reaction.
Stoichiometric calculations are used in various fields of chemistry, such as chemical manufacturing, analytical chemistry, and environmental chemistry. These calculations help determine the limiting reactant, theoretical yield, and actual yield of a reaction.
To balance chemical equations, coefficients are added to the reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation.
Mole Concept
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
The mole is a convenient unit for expressing the amount of a substance because it allows us to relate the mass of a substance to the number of atoms or molecules it contains.
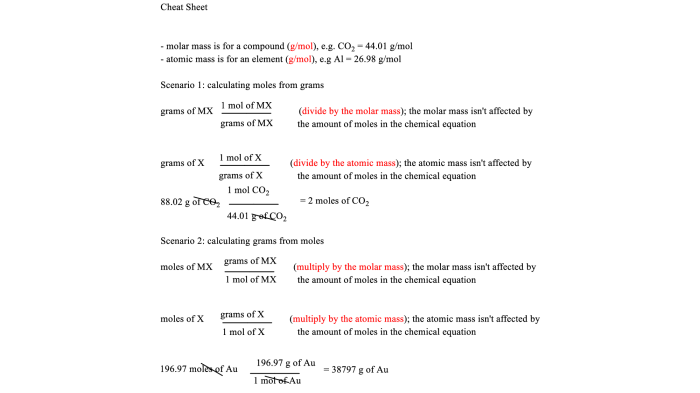
The relationship between moles and mass is given by the following equation:
moles = mass / molar mass
where molar mass is the mass of one mole of a substance.
Stoichiometric Calculations, Chapter 11 stoichiometry study guide
Stoichiometric calculations are used to determine the amount of reactants or products that are involved in a chemical reaction. These calculations are based on the balanced chemical equation for the reaction.
To perform a stoichiometric calculation, we need to know the mole ratio between the reactants and products. This ratio is obtained from the coefficients in the balanced chemical equation.
Once we know the mole ratio, we can use it to calculate the amount of reactants or products that are involved in the reaction.
Helpful Answers
What is the significance of stoichiometry in chemistry?
Stoichiometry provides a quantitative framework for understanding chemical reactions, enabling the prediction of product yields, reactant requirements, and the overall efficiency of chemical processes.
How does the mole concept facilitate stoichiometric calculations?
The mole concept establishes a bridge between the macroscopic and microscopic worlds, allowing chemists to relate the mass of a substance to the number of its constituent particles, simplifying stoichiometric calculations.
What are the key steps involved in balancing chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each reactant and product to ensure that the number of atoms of each element is identical on both sides of the equation, reflecting the law of conservation of mass.