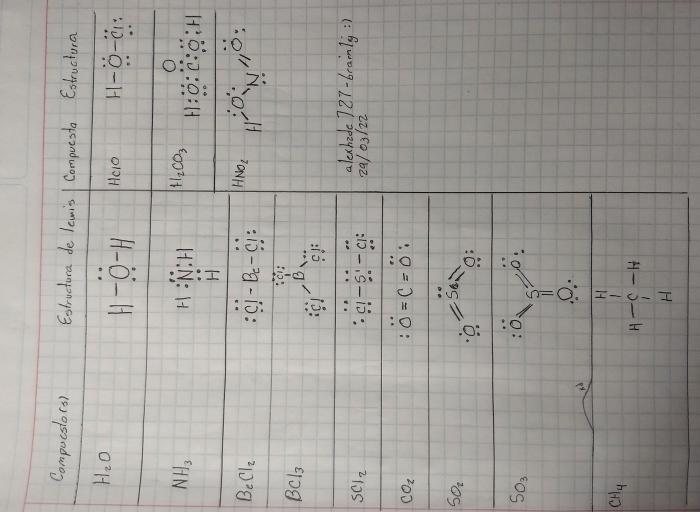
Classify each of the following compounds as ionic or molecular. Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions are held together by electrostatic forces. Molecular compounds are formed when two or more nonmetals share electrons.
The resulting molecules are held together by covalent bonds.
The table below lists several compounds and their classifications. The “Formula” column gives the chemical formula of each compound. The “Ionic or Molecular” column indicates whether the compound is ionic or molecular. The “Explanation” column provides a brief explanation of the classification.
Ionic and Molecular Compounds: Classify Each Of The Following Compounds As Ionic Or Molecular

Compounds are substances composed of two or more different elements chemically combined in fixed proportions by mass. They can be classified into two main categories: ionic and molecular compounds.
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions are held together by electrostatic attraction. Molecular compounds, on the other hand, are formed when two or more nonmetals share electrons. The resulting molecules are held together by covalent bonds.
Classification of Compounds, Classify each of the following compounds as ionic or molecular
| Compound | Formula | Ionic or Molecular | Explanation |
|---|---|---|---|
| Sodium chloride | NaCl | Ionic | Sodium is a metal and chlorine is a nonmetal. Sodium loses one electron to chlorine, forming Na+ and Cl- ions, which are held together by electrostatic attraction. |
| Water | H2O | Molecular | Hydrogen and oxygen are both nonmetals. They share electrons to form a covalent bond, resulting in a molecule of water. |
| Carbon dioxide | CO2 | Molecular | Carbon and oxygen are both nonmetals. They share electrons to form covalent bonds, resulting in a molecule of carbon dioxide. |
| Potassium iodide | KI | Ionic | Potassium is a metal and iodine is a nonmetal. Potassium loses one electron to iodine, forming K+ and I- ions, which are held together by electrostatic attraction. |
Properties of Ionic and Molecular Compounds
| Property | Ionic vs. Molecular |
|---|---|
| Solubility in water | Ionic compounds are generally soluble in water, while molecular compounds are generally insoluble. |
| Electrical conductivity | Ionic compounds conduct electricity when dissolved in water or melted, while molecular compounds do not. |
| Melting point | Ionic compounds have high melting points, while molecular compounds have low melting points. |
| Boiling point | Ionic compounds have high boiling points, while molecular compounds have low boiling points. |
Examples of Ionic and Molecular Compounds
- Ionic compounds: NaCl, KCl, CaO, MgCl2, Na2SO4
- Molecular compounds: H2O, CO2, CH4, NH3, C6H12O6
Questions and Answers
What is the difference between an ionic and a molecular compound?
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions are held together by electrostatic forces. Molecular compounds are formed when two or more nonmetals share electrons. The resulting molecules are held together by covalent bonds.
What are some examples of ionic compounds?
Some examples of ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium oxide (CaO).
What are some examples of molecular compounds?
Some examples of molecular compounds include water (H2O), methane (CH4), and carbon dioxide (CO2).