Embark on a journey into the fascinating world of chemistry with our meticulously crafted Stoichiometry and Percent Yield Worksheet. This comprehensive resource empowers you to delve into the fundamental principles of stoichiometry and percent yield, equipping you with a solid foundation in these essential chemical concepts.
Through a series of engaging exercises and thought-provoking questions, you will explore the intricacies of chemical reactions, mastering the art of predicting product quantities and evaluating reaction efficiency. Prepare to unravel the mysteries of stoichiometry and percent yield, unlocking a deeper understanding of the chemical world.
Stoichiometry and Percent Yield Concepts: Stoichiometry And Percent Yield Worksheet
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants and products involved in a given reaction. Percent yield is a measure of the efficiency of a chemical reaction, expressed as the ratio of the actual yield to the theoretical yield multiplied by 100%.
Stoichiometry and percent yield are essential concepts in chemistry, as they allow us to understand and control chemical reactions. They are used in various fields, including chemistry, biochemistry, and environmental science.
Stoichiometry Calculations, Stoichiometry and percent yield worksheet
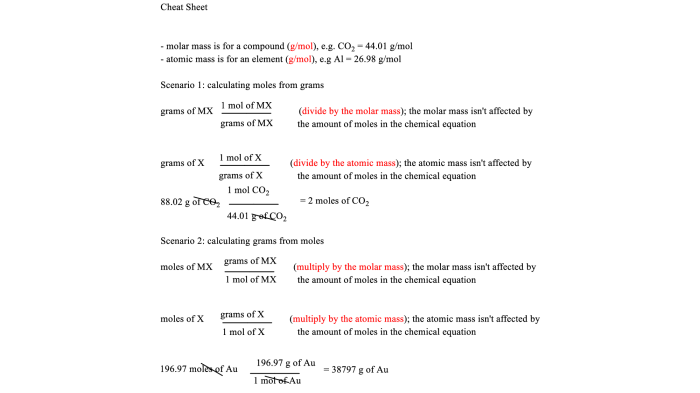
Stoichiometry calculations involve using the mole concept and balanced chemical equations to determine the quantitative relationships between reactants and products. The steps involved in performing stoichiometry calculations include:
- Balancing the chemical equation.
- Converting the given mass or volume of a reactant or product to moles using the molar mass or molar volume.
- Using the stoichiometric coefficients in the balanced equation to determine the mole ratio between the reactant and product.
- Calculating the moles of the product formed or the moles of the reactant required.
- Converting the moles of the product or reactant back to mass or volume using the molar mass or molar volume.
Percent Yield Calculations
Percent yield calculations involve using the actual yield and theoretical yield of a reaction to determine the efficiency of the reaction. The steps involved in calculating percent yield include:
- Determining the theoretical yield of the reaction using stoichiometry calculations.
- Measuring the actual yield of the reaction experimentally.
- Calculating the percent yield using the formula: Percent Yield = (Actual Yield / Theoretical Yield) x 100%.
FAQ Section
What is the significance of stoichiometry in chemistry?
Stoichiometry serves as the cornerstone of chemical reactions, allowing us to predict the exact quantities of reactants and products involved. It ensures balanced chemical equations, enabling us to optimize reaction conditions and maximize product yield.
How does percent yield provide insights into reaction efficiency?
Percent yield measures the actual yield of a reaction compared to its theoretical maximum. It reveals the efficiency of the reaction process, highlighting potential losses or inefficiencies. By analyzing percent yield, we can identify areas for improvement and optimize reaction conditions.